PIC/S GMP Compliant
Facility and Services
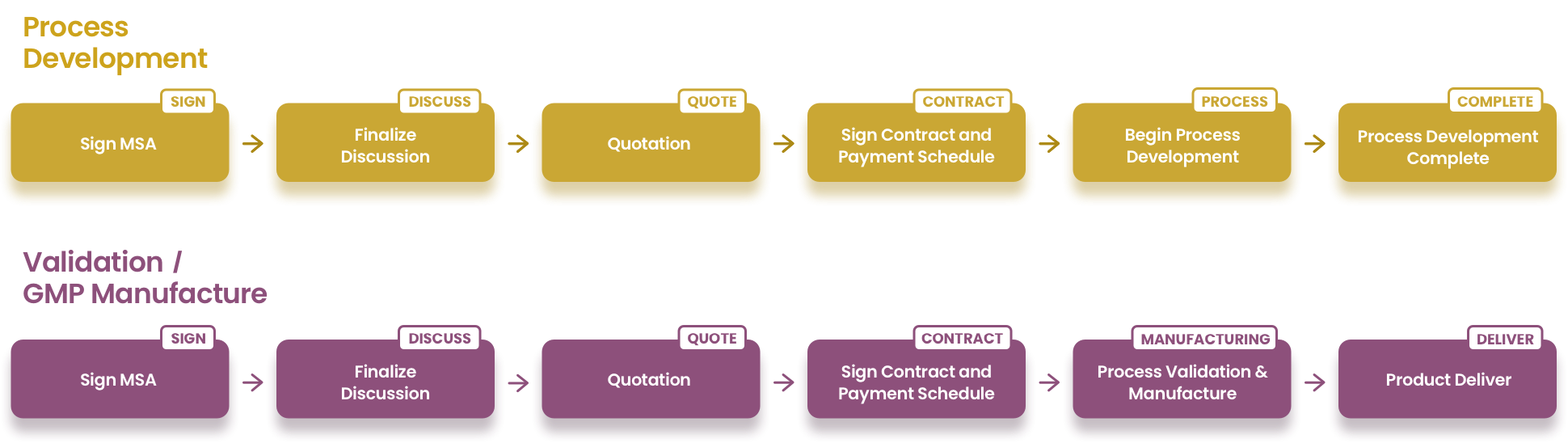
HKIB operates a state-of-the-art Current Good Manufacturing Practice (cGMP) production facility for advanced therapy products. As the leading manufacturer in Hong Kong, HKIB can translate your academic cell therapy protocols to GMP, process development and the associated documentation required for GMP manufacturing for the use in clinical trials. Our manufacturing processes are fully customizable and scalable to support your needs and our cGMP suites and labs are designed to support multiple stages of clinical development.
Cleanrooms
Different products required different manufacturing needs. Our PIC/S GMP compliant facility has 2 Grade B cleanrooms and 4 Grade C cleanrooms equipped with validated production equipment for Advanced Therapy Product manufacture. Each room is supplied with central synthetic air, carbon dioxide and nitrogen to accomplish the need of different manufacturing process for different products.
The facility is equipped with Laminar Flow Passbox and VHP sterilization equipment for material transit into different level of cleanliness.

Production Equipment
Miltenyi CliniMACS Prodigy
The CliniMACS Prodigy is a closed system automated cell processing device offering advanced integrated solutions to streamline cell processing workflows – from cell separation through cell culture to formulation of the final product.
Terumo TSCDII Tube Welder
Terumo Tube Welder is a closed-system device that can be used to connect PVC tubing in any combination of wet and dry.
Baker Class II Biological Safety Cabinet
Two at grade C and one at grade B cleanroom that laminar air supplied by the BSC's HEPA filters meet Class 5 conditions per ISO 14644-1 and 2.
Pall Palltronic® Filter Integrity Test Device
Filter integrity test device addresses GMP manufacturing demands. It supports critical filtration steps and batch release with reliable and accurate filter testing. The device fully fulfils the regulatory requirements regarding data integrity with it access management and audit trail features.
Cytiva VIA Freeze Control Rate Freezer
Control Rate Freezer is a liquid nitrogen-free Freezer that combining customizable freezing profiles, electronic control systems, and a conduction cooling method to optimize GMP-compliant for cryopreservation of our finished products.
CryoPLUS 1 Liquid Nitrogen Storage System
Liquid Nitrogen Storage System provides an ultralow temperature (below -130°C) for cell storage without loss of cell viability. With Control panel mounted on top for convenient access and easy touchpad programming. Also equipped with liquid level sensor and alarm contacts for remote monitoring of storage tank condition.
MVE Vapor Shipper
Vapor Shipper Series is designed for the safe transportation of biological samples at cryogenic (-150°C or colder) temperatures and utilize an advanced QWick Charge material that charges with liquid nitrogen in fewer than two hours, providing the capacity for same-day vapor shipping.
Precision Balance
Offer higher weighing capacities than their analytical counterparts. In addition to high capacity and durability, the balances have advanced features that include dosing, counting, percentage weighing, mixing, totalizing, animal weighing, free factor, density determination, statistics, peakhold, checkweighing, mass unit conversion, and pipette smart test.








Analytical Equipment
Miltenyi MACSQuant® Analyzer 10
A benchtop flow cytometer for highly sensitive, multi-parameter cell analysis. It provides fully automated instrument setup using pre-set calibration and compensation programs, as well as automated startup, shutdown, and cleaning cycles for hassle-free instrument housekeeping.
StepOnePlus PCR System
StepOnePlus PCR System is a 96-well Real-Time PCR instrument. It can be setup in a variety of configurations and comes ready to use, out of the box, with intuitive data analysis and instrument control software.
Nanodrop One
Nanodrop One is a Microvolume UV-Vis Spectrophotometer. It can improve the measurement accuracy and contaminant identification. Quantify and qualify DNA, RNA, and protein samples in seconds with only 1-2 µL, and obtain full-spectral data before you decide to use samples in downstream applications.
Eppendorf ® Centrifuge 5810R
Centrifuge 5810R, a benchtop centrifuge for separating cells from buffer and other suspension solutions during QC experiments. With its renowned quality and reliability, it offers the solution for medium to high-throughput applications requiring speeds of up to 20,913 x g (14,000 rpm).
Endosafe-Nexgen PTS
The Endosafe® nexgen-PTS™ is a rapid, point-of-use handheld spectrophotometer that uses USP/BET-compliant disposable cartridges for accurate, convenient, and real-time endotoxin testing, glucan concentration determination, and Gram identification.
Streamline® PCR Cabinet
Esco Streamline® PCR Cabinet (SCR) provides a compact and low footprint environment for handling reagents such as PCR master mixes. It is equipped with simple rocker switches to control blower, light, and UV. The HEPA-filtered air guarantees a contaminant-free environment for reliable PCR results.






Get In Touch
We look forward to hearing from you. Get in touch to bring your development project to life or become a valued technology collaborator.